Our Categories
Providing technical consultancy services for biosimilar products requires a deep understanding of the biopharmaceutical industry, regulatory requirements, and the specific challenges associated with developing biosimilars. Here is a detailed description consultancy services we are typically offered
Initial Assessment
The process begins with an initial assessment of the client's needs and goals. This involves understanding the client's specific biosimilar product, its intended market, and the stage of development or production they are currently at.
Regulatory Guidance
One of the key aspects of providing consultancy for biosimilar products is helping clients navigate the complex regulatory landscape. Consultants assist in interpreting and complying with regional and international regulations.
Product Development Strategy
Consultants work closely with clients to develop a strategic plan for the biosimilar product. This includes defining the target product profile, selecting the appropriate reference product, and outlining the development pathway.
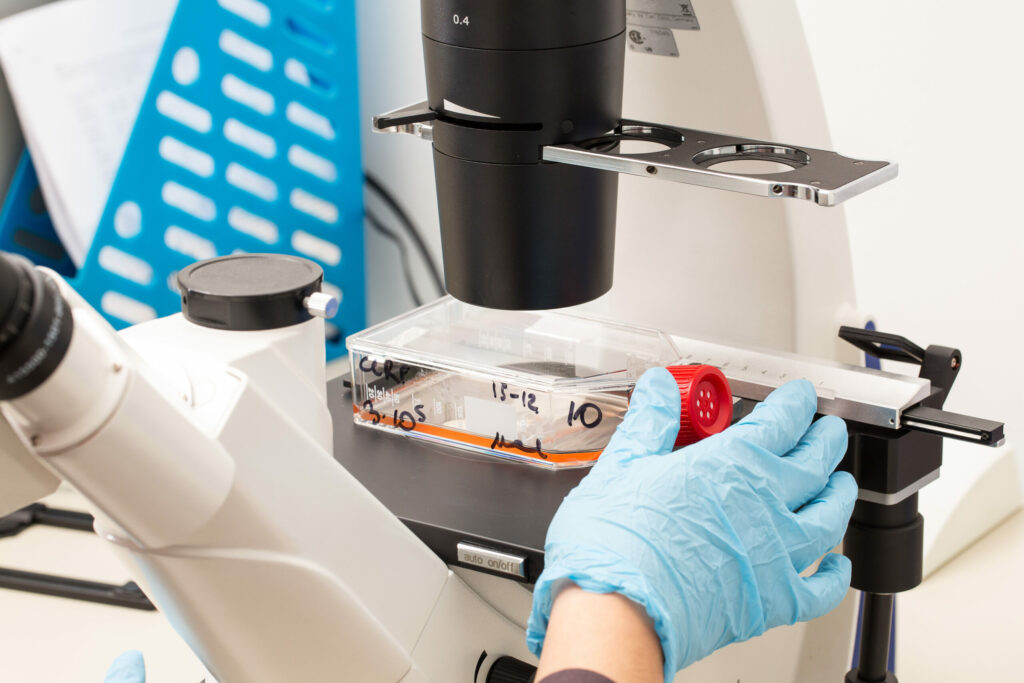
Analytical Method Development
Consultants assist in the development and validation of analytical methods used to characterize the biosimilar and demonstrate similarity to the reference product. This includes physicochemical and biological assays.
Quality Control and Assurance
Ensuring the quality and consistency of biosimilar products is paramount. Consultants help establish quality control and quality assurance systems, including GMP (Good Manufacturing Practices) compliance.


Clinical Development
If clinical trials are required, consultants provide guidance on designing and conducting these trials. This includes designing appropriate study protocols and addressing ethical and safety considerations.
0+
Awards Win
0K
Happy Customers
0%
Satisfaction
0+
Years of experience